Not one of us undergoes a surgical procedure with the hope of walking out the door with an infection along with the medical bill. Unfortunately, incidents of infections from healthcare facilities do occur, and opportunities for increased incidence are growing with the surge of ambulatory surgical centers, or ASCs, throughout the United States.
Oftentimes, ASCs are multidisciplinary, focusing on higher efficiency of care through flexible use of space and resources. As growth continues to rise, situations like Toxic Anterior Segment Syndrome, which spiked within the last decade, may occur more often as the complexity and breadth of surgical services offered is on the increase. However, significant measures to reduce risks, lower costs and improve quality of care can be realized with the adoption of “process-driven” design and planning, regardless of a project’s size and budget.
Recently, Array Architects worked with a healthcare provider to update an established ASC that was in need of improving efficiency and outcomes. This particular ASC is part of an outpatient medical center with a host of specialty services, and has served a group of multi-disciplinary clinical and facility staff for over 30 years. The group was eager to share its knowledge and wish list, and more than ready to embrace the latest industry guidance and best practices.
With an addition of a procedure room dedicated to ophthalmology, it is not surprising that one of the top design priorities was to modify the sterile processing department to include safe processing of eye instruments. Ideally, intraocular instruments would be cleaned separately from nonophthalmologic surgical instruments, as recommended by The American Academy of Ophthalmology and the American Society of Cataract and Refractive Surgery to reduce risks of TASS outbreaks similar to those in 2005-06.
As renovation projects, traditional limitations such as footprint, budget, operational efficiency and aggressive schedule are top of mind. An initial assessment of conditions indicated that lack of physical separation resulted in multiple opportunities for cross-contamination. To date, the healthcare provider/organization had successfully managed the risks during the outpatient center’s operating history using heightened procedural controls. However, to effectively adopt and maximize the benefits of the latest guidance without a duplicate SPD department, a limited redesign of the SPD area was advocated by engaging the clinical group in a process-centered, risk-based approach.
The not-so-dirty details
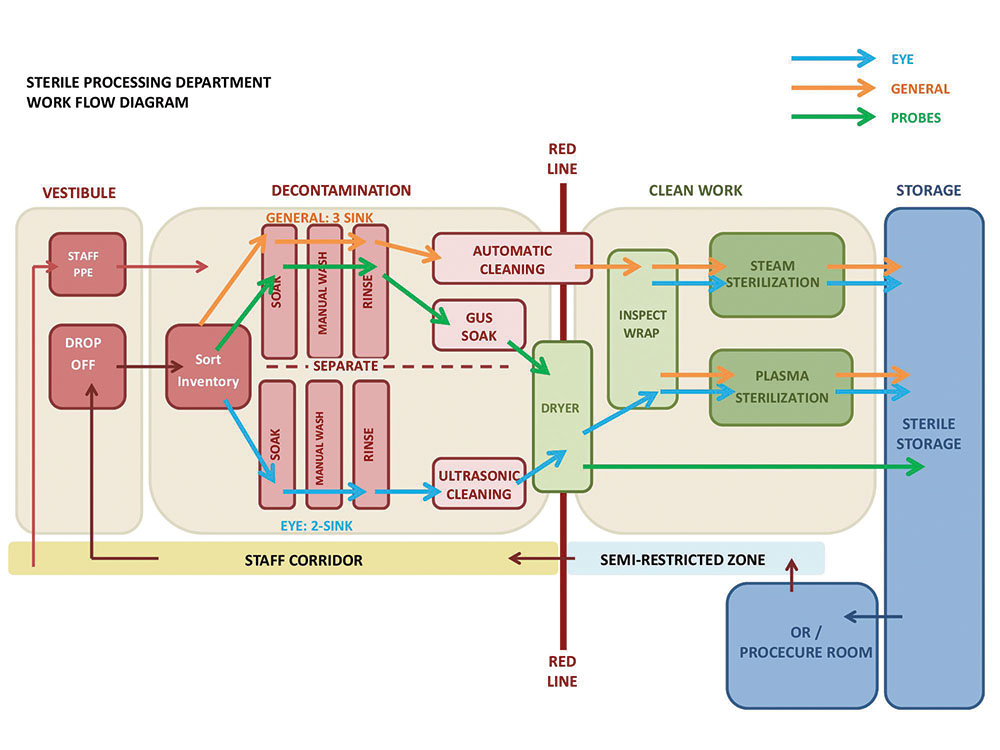
Modern sterilization of instruments is typically completed in multiple steps, contained in three distinct areas: soil work area, clean work area and sterile storage. Soiled instruments are dropped off at the soil work area for decontamination where instruments are sorted, washed and cleaned manually and/or mechanically and disinfected as appropriate. Next, clean instruments are inspected and assembled in sets, in preparation for sterilization if required. Packaged instruments then undergo the sterilization process to destroy harmful microorganisms. Finally, sterile packages of instruments are transferred to sterile storage areas, ready to be issued. Each instrument is processed through a linear path from soiled to clean then sterile, but a variety of equipment is utilized and shared at each stage to meet requirements of individual instruments.
As the workflow of instrument processing was studied in this particular outpatient facility, it was discovered that a vast majority of instruments required very similar paths. After sorting, all reusable instruments were manually washed at cleanup sinks as a first step of processing, then further cleaned and/or disinfected in one of three machines. In a clean work area, all instruments requiring sterilization were inspected and wrapped, then sterilized in steam or plasma sterilizers prior to storage. While there is not a lot of equipment in this particular department, the workflow diagram highlighted several potential risks in the sterile processing approach that could be addressed. These included:
1. Lack of physical separation between intraocular and non-ophthalmologic surgical instruments
2. Splashing of water during manual cleaning process poses the greatest risk of contamination
3. Instruments unnecessarily cross paths in the decontamination area
It was also discovered that eye instruments follow a distinct processing path in the decontamination area only, where instruments are dirtiest and quality is mostly likely to suffer if debris were inadvertently shared with other instruments.
With a clear understanding of this department’s detailed workflow, the team was able to quickly create a diagram to illustrate how a limited separation and careful positioning of cleanup sinks in decontamination can create an effective and safe workflow. In this concept, eye instruments complete the entire two-step manual and ultrasonic cleaning process with little risk of cross contamination. Once cleaned, all instruments are then inspected, packaged and sterilized alongside other instruments, maximizing efficiency of staff and equipment at the clean work area of the department.
After receiving input from all stakeholders, the group worked together to develop a detailed layout utilizing actual equipment specifications and facility dimensions. The clinical and sterile processing team was immediately excited about this design, as desired separation between general and eye instruments was achieved without any impact on departmental operations and staffing. The facility team was satisfied to meet the latest industry guidance, while limiting major renovation scope to meet a fast-paced project schedule.
Small steps can make a big impact
Healthcare design, whether for hospitals or outpatient clinics, results in long-term and daily changes. It is even more critical for architects and designers to listen carefully to clients’ needs and to effectively understand perspectives. Only then can we discover the most impactful opportunities to enhance a client’s ability to deliver care while meeting the project’s financial and schedule goals. Even a seemingly minor facility renovation project can present significant opportunities for repeatable improvement on clinical operations and safety, as well as renew patient satisfaction.